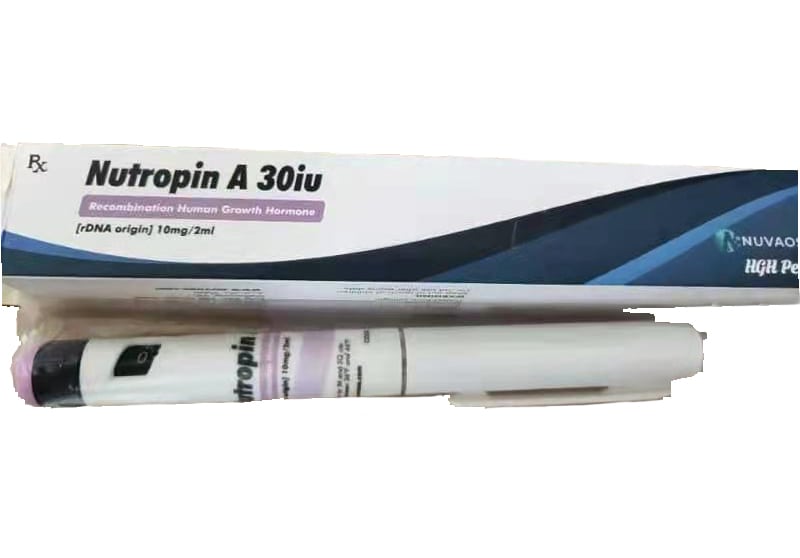
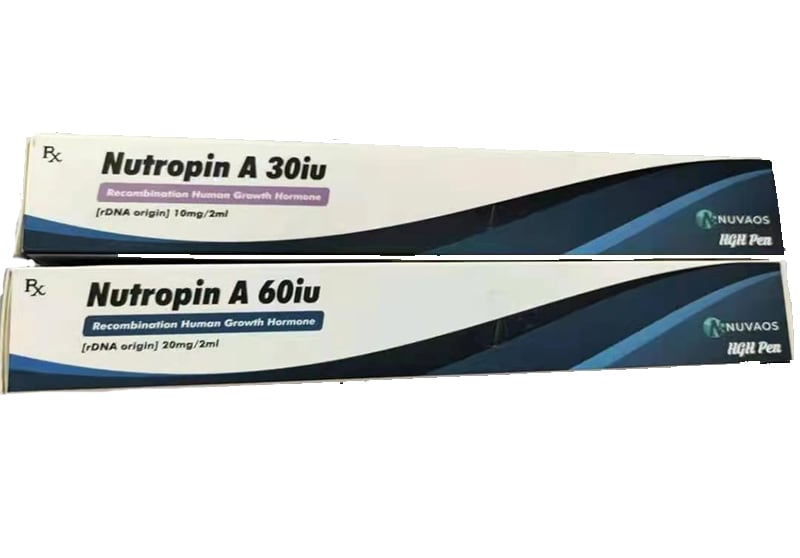
Nutropin AQ 30iu Pre Mixed 10mg 2ml 1 pen box – High Quality For Bodybuilding
- Country of origin: —
- Manufactured since: 2008 ?C Present
- Package :30iu Pre Mixed 10mg 2ml 1 pen box
- Price: $1.5 ?C $5 per IU
- Minimum Order:1 kits
- Storage:2 ℃– 8 ℃ in room temperature
- Delivery: 5-15 days
100% delivery
- Satisfaction Guaranteed
- No Hassle Refunds
- Secure Payments
Description
Nutropin [somatropin (rDNA origin) for injection] is a human growth hormone (hGH) produced by recombinant DNA technology. Nutropin has 191 amino acid residues and a molecular weight of 22,125 daltons. The amino acid sequence of the product is identical to that of pituitary-derived hGH. Nutropin may contain not more than fifteen percent deamidated GH at expiration. The deamidated form of GH has been extensively characterized and has been shown to be safe and fully active.
Nutropin is one HGH injection that is FDA approved to treat growth deficiencies. Nutropin is also used to treat other conditions that can lead to an improvement in quality of life. Of course, you should only undertake the use of Nutropin or any other HGH injection under the direction and guidance of your doctor. Right now, read on to learn a little more about Nutropin.
You must be logged in to post a review.


Reviews
There are no reviews yet.